| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Striking the Balance: Why Optimal Body pH Matters and How to Achieve It
Follow TIS on Twitter: @Truth_is_Scary & Like TIS of Facebook- facebook.com/TruthisScary
When I was 22 years old, I felt drained and tired, had little motivation to do anything, and experienced a general lack of energy. My hair had changed from shiny, full, and curly to falling out and less full, dry, and straight, even strawy. Something was amiss. Curious about the cause, I saw a general practitioner. He told me I was in good health, and that everything was just fine. What he said and how I felt did not match. If everything was well, why did I not feel it?
To cut a long story short, it was not until many years later when I tried wheatgrass juice that this changed dramatically. What happened? Seemingly negligibly little. I had changed nothing except for drinking three wheatgrass shots every other day, within one week. The effect was amazing. It gave a powerful reminder of what it’s like to wake up wide awake in the morning, ready to excitedly jump out of bed and greet a brand new day – a feeling I hadn’t experienced for nearly two decades but recognized instantly. It was awesome and familiar, along the lines of “wherever have you been all this time?!”
From then on, fresh wheatgrass juiceformed a daily part of my life. A 30 ml shot equates the goodies present in about one kilogram of vegetables. Still unaware of why wheatgrass juice had this effect, I enjoyed the raised energy levels a great deal regardless. Curious once more, I looked into the subject. This article shares what I found. It’s amazing to think this solution could have emerged years and years ago, had the idea occurred to my GP to test pH. I don’t say this to blame him, but rather to illustrate a point another MD made in her book: that testing body pH is rarely part of general medical practice (Kraske 2005). Yet, it’s such a fundamental component of a healthy body and mind, and one easily addressed at home (given no illness is present).
Chemistry 101 – Acid, Base, pH – What were they again?
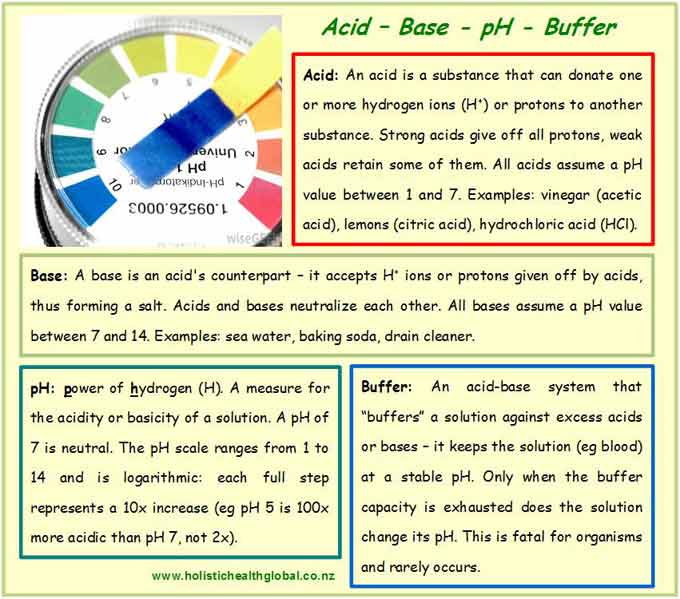
In a nutshell, an acid donates hydrogen ions (H+) or protons, and a base receives them (Box 1). Ions are charged atoms or particles. Bases also contain one or more hydroxyl groups (OH–). Acids and bases neutralize each other. Both are important for our metabolism. In the body, when acids and bases react, they produce neutral salts and water. These salts are easily excreted (e.g. sweat) and pose no issue.
The logarithmic pH scale offers a measure of the acidity or basicity of a substance. The acronym pH stands for the power of hydrogen (H) (Box 1). It measures the concentration of hydrogen ions in one liter of aqueous solution: the more H+ ions, the stronger the acid and the lower the pH reading. The pH scale ranges from 1 to 14. Substances behave neutral (neither acidic nor basic, i.e. the solution contains equal amounts of H+ and OH– ions) around pH 7. Higher pH values (above 7) reflect a stronger basicity. Hydrochloric acid (HCl) in our stomach, for example, is pH 1, whereas our blood maintains a constant pH between 7.34 and 7.45. Outside this narrow range, human life would cease fairly quickly. People with acidosis (a constant blood pH below 7.35) require intensive medical care. Alkalosis, a constant blood pH above 7.45, rarely occurs but is equally serious. The words basic and alkaline are often used interchangeably, denoting a pH above 7.
Source: http://truthisscary.com/2015/05/striking-the-balance-why-optimal-body-ph-matters-and-how-to-achieve-it/