| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Particle Physics – HC Unit geometry – why do the numbers work?
just recently, I did a post about the geometric nature of the first two Nobel Gases, Helium and Neon.
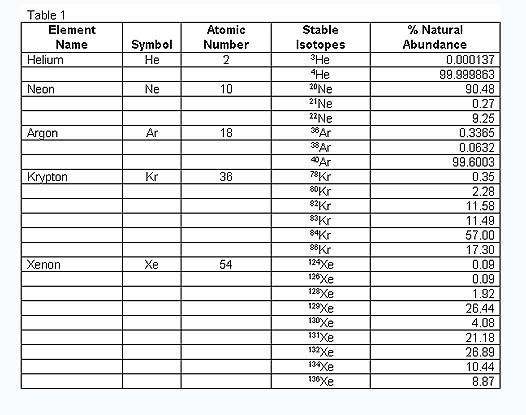
From the above table, you can see that Helium has an atomic number of two, Neon has an atomic number of 10. In classical electron theory, this means Helium has one full two-electron shell; Neon has two full shells, the first two-electron, the second eight-electron.
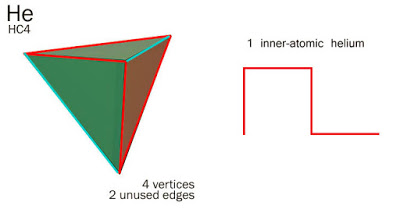
But, my HC Unit makes Helium by putting FOUR HC Units in a ring, feeding each other, ninety degrees out of phase with NO NET CHARGE or no recordable discharge. In geometric terms, this means that Helium wobbles between a square and a tetrahedron. Vertices 1 and 3, and vertices 2 and 4, interact to produce the 'two-electron first full shell'.
You'll see that the 'square' or 'tetraheral' 4-HC helium is a series of 90-degree angles if laid flat in the series + 0 – 0…
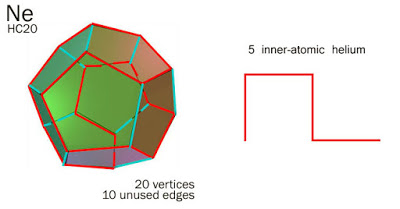
We can use this to make HC20-Neon, there will be five of these laid flat HC4-heliums, chained on the surface of a dodecahedron to make Neon. The ten unused edges are the two full shells, 2 + 8.
But it's a lot easier than this, geometrically…
Helium = 4 HC Units = 1 Helium.
Neon = 20 HC Units = 5 Heliums.
Argon = 36 HC Units = 9 Heliums.
Krypton = 72 HC Units = 18 Heliums.
Xenon = 108 HC Units = 27 Heliums.
It works, but what are we seeing here?
Source: http://mikephilbin.blogspot.com/2016/10/particle-physics-hc-unit-geometry-why.html