| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
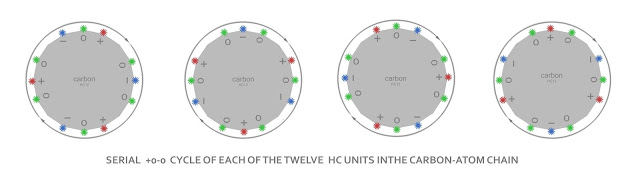
HC Unit – Carbon cycle – four spinning valences
HC Unit, I don't even really know what that means, but four of them seem to make a non-excessing (or no-net-charge) Helium atom, the first Nobel gas. Twenty of them seem to make a non-excessing (or no-net-charge) Neon atom. Twelve of them therefore have four valence gaps in their ring or cycle… i.e. Carbon 12.
I suspect that the 'atomic structure' of the larger-ring atoms is based on the arrangement of the previous 'nobel' atoms otherwise seen as four kinks in the HC12 chain i.e. Carbon would have a 'structural' or 'virtual' +0-0 Helium at the core of its twelve with the eight remaining outer HCs-in-the-chain offering the valence of what we call four outer shell electrons in particle-speak.
We make the 'virtual helium' in the HC12 chain by taking the first HC of each three-HCs in the twelve chain, this gives us the classical +o-o of Helium while retaining the cycle of the twelve. Each of the remaining eight HCs in the twelve is paired off as either a o- or a o+ standing out from the 'atom'.
Source: http://mikephilbin.blogspot.com/2016/12/hc-unit-carbon-cycle-four-spinning.html