| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Striking the Balance: Why Optimal Body pH Matters and How to Achieve It
17th May 2015
By Katrin Geist
Guest Writer for Wake Up World
When I was 22 years old, I felt drained and tired, had little motivation to do anything, and experienced a general lack of energy. My hair had changed from shiny, full, and curly to falling out and less full, dry, and straight, even strawy. Something was amiss. Curious about the cause, I saw a general practitioner. He told me I was in good health, and that everything was just fine. What he said and how I felt did not match. If everything was well, why did I not feel it?
To cut a long story short, it was not until many years later when I tried wheatgrass juice that this changed dramatically. What happened? Seemingly negligibly little. I had changed nothing except for drinking three wheatgrass shots every other day, within one week. The effect was amazing. It gave a powerful reminder of what it’s like to wake up wide awake in the morning, ready to excitedly jump out of bed and greet a brand new day – a feeling I hadn’t experienced for nearly two decades but recognized instantly. It was awesome and familiar, along the lines of “wherever have you been all this time?!”
From then on, fresh wheatgrass juiceformed a daily part of my life. A 30 ml shot equates the goodies present in about one kilogram of vegetables. Still unaware of why wheatgrass juice had this effect, I enjoyed the raised energy levels a great deal regardless. Curious once more, I looked into the subject. This article shares what I found. It’s amazing to think this solution could have emerged years and years ago, had the idea occurred to my GP to test pH. I don’t say this to blame him, but rather to illustrate a point another MD made in her book: that testing body pH is rarely part of general medical practice (Kraske 2005). Yet, it’s such a fundamental component of a healthy body and mind, and one easily addressed at home (given no illness is present).
Chemistry 101 – Acid, Base, pH – What were they again?
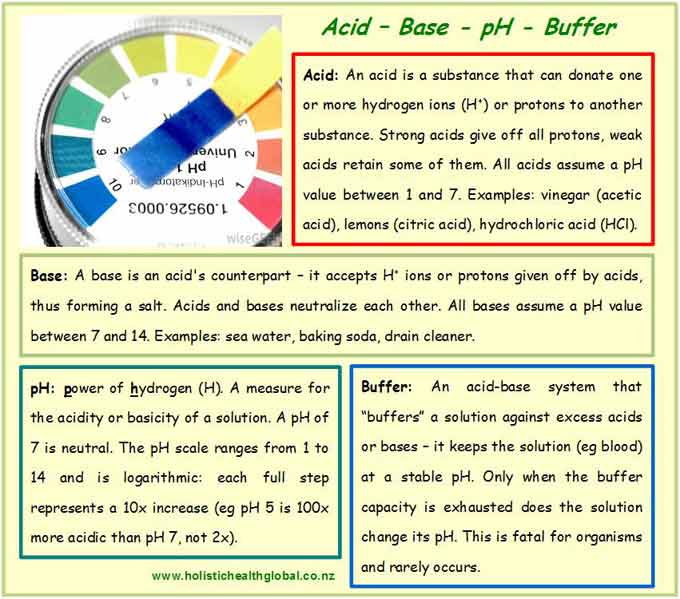
In a nutshell, an acid donates hydrogen ions (H+) or protons, and a base receives them (Box 1). Ions are charged atoms or particles. Bases also contain one or more hydroxyl groups (OH–). Acids and bases neutralize each other. Both are important for our metabolism. In the body, when acids and bases react, they produce neutral salts and water. These salts are easily excreted (e.g. sweat) and pose no issue.
The logarithmic pH scale offers a measure of the acidity or basicity of a substance. The acronym pH stands for the power of hydrogen (H) (Box 1). It measures the concentration of hydrogen ions in one liter of aqueous solution: the more H+ ions, the stronger the acid and the lower the pH reading. The pH scale ranges from 1 to 14. Substances behave neutral (neither acidic nor basic, i.e. the solution contains equal amounts of H+ and OH– ions) around pH 7. Higher pH values (above 7) reflect a stronger basicity. Hydrochloric acid (HCl) in our stomach, for example, is pH 1, whereas our blood maintains a constant pH between 7.34 and 7.45. Outside this narrow range, human life would cease fairly quickly. People with acidosis (a constant blood pH below 7.35) require intensive medical care. Alkalosis, a constant blood pH above 7.45, rarely occurs but is equally serious. The words basic and alkaline are often used interchangeably, denoting a pH above 7.
Naturally acidic and alkaline organs and minerals
The stomach, skin, and vagina are examples of naturally more acidic organs, whereas salivary glands, pancreas, gallbladder, liver, and intestines require and create more alkaline environments. Acidic minerals: sulphur, phosphorus, chloride, iodine, and silica. Alkalizing minerals: compounds containing potassium, calcium, magnesium, iron, and sodium. These need to be present in every cell to neutralize acidic metabolites. Nowadays, many people lack sufficient supply of these minerals.
In order to maintain proper blood pH, the body uses various buffer systems which allow it to temporarily “soak up” excess acids. It follows that these buffer systems require careful maintenance, lest they be depleted, forcing the body to use its own substance to neutralize acids – a less than ideal and all too common scenario in Western populations.
What’s a buffer? What roles do breathing and hydration play?
A buffer is an acid-base system that maintains a highly stable pH in aqueous solution (Box 1). No matter how much acid you add, for example, the pH will not change. That is, until the buffer becomes exhausted, at which point the solution will eventually turn acidic. Ideally, though, this point is never reached and the pH maintained. Addition or evaporation of water also do not markedly affect the pH of a buffer solution. The blood’s carbonic acid – bicarbonate buffer is a good example: our cells’ metabolism consistently produces carbonic acid,H2CO3. Carbonic acid and water react to bicarbonate and a hydronium ion: H2CO3 + H2O ? HCO3– + H3O+. This equation forms our carbonic acid – bicarbonate buffer system. Add an acid and it reacts with the base (HCO3–), add a base and it reacts with the acid (H2CO3) – the pH remains stable as long as enough buffer molecules are present. Carbonic acid also dissociates to H2O and CO2,which we then exhale through the lungs. This is why deep breathing is so beneficial. Not only does it directly oxygenate our cells, it also releases CO2, thus helping to maintain pH. Since we also exhale bicarbonate, there is no net purging of acids via the lungs. This task falls to our kidneys, which eliminate excess acids in form of the ammonium ion (NH4+). If acids were excreted directly, cells in the kidneys and urinary tract would suffer acidity damage. In order to successfully rid the body off excess acids, kidneys require enough water – that is why drinking enough is vitally important! It directly nourishes cells, supports the body’s detox systems, and helps maintain its acid-base balance. It may well be the simplest remedy to employ, alleviating many a symptom. Of course, the water you drink should be of high quality…I will offer a separate article about water later. It is the number one food, and for good reason. A simple test to see if you’re well hydrated: pinch a bit of skin on the back of your hand and let go. It should immediately smooth out again. If the skin fold persists for more than three seconds, you’re dehydrated.
Why is the acid-base balance important?
If the body lacks a sufficient base supply to neutralize acids, they can be temporarily stored in connective tissues and muscles to await neutralization. If this step does not occur, however, problems may eventually arise due to a chronic acid overload (see below). High acid levels in the blood and other body fluids disturb ana- and catabolic pathways (the building and breaking down of molecules). Cellular function depends on the right milieu. For example, enzymes (catalytic proteins) rely on a specific pH for optimal form and function: the stomach requires high acidity (pH 1 – 3) to break down proteins, whereas the pancreas works best at pH 10, and the intestine between pH 6 and 7. Cell membranes, distribution of electrolytes, and connective tissue function all depend on the right cellular environment (including pH levels), as does the blood and really all body fluids. Our heart circulates c.7500 liters of blood through c. 96.500 km of blood vessels every day. Blood reaches and communicates with all organs and tissues, transporting oxygen and nutrients to all cells, carbonic acid to the lungs, and waste metabolites to the kidneys. Lack of water and acid overload both reduce its flowing ability, preventing blood from reaching the smallest capillaries – consequently, those tissues may turn anaerobic (since no oxygen is supplied, they must switch to functioning without it, a sub-ideal situation, yielding less energy and encouraging pathogens and cancer cell growth).
The acid-base interplay is of paramount importance for all other metabolic events in the body. It forms the basis for good health and quick recovery from illness. When the body has enough bases to neutralize acids, we are in balance.
Previous articles by Katrin Geist:
- The FAT Facts: Butter vs Margarine
- The Power of Convictions and How They Shape Our Lives
- 10 Significant Reasons Why Regularly Drinking Green Tea Is An Awesome Healthy Living Habit!
- Cradle to Cradle Design – How a Biochemist and an Architect Are Changing the World
- Truly Healing From Cancer and Preventing It Altogether
- How to Lose Your Mind and Create a New One
- Research Shows Promising Effects Treating Advanced Cancer with Light Frequencies
- Depression & Anxiety: Discover 3 Powerful, Drug-Free Ways that Help Thousands, Naturally
- The Power of Suggestion – Are You Asking the Right Questions?
-
Follow Wake Up World On…
[FACEBOOK]: http://www.facebook.com/joinwakeupworld (An interactive community of over 2,000,000)
[PINTEREST]: http://pinterest.com/wakeupword/
[TWITTER]: http://twitter.com/joinwakeupworld
[YOUTUBE]: http://www.youtube.com/joinwakeupworld
[GOOGLE PLUS]: https://plus.google.com/112452105795129310867/posts
[WEBSITE]: http://wakeup-world.com






thanks !