

| Visitors Now: | |
| Total Visits: | |
| Total Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
S05E03: The Pulled Groin Extrapolation
An now, the must-watch exciting conclusion of the axion calculation saga on the whiteboards.
Last week we saw two episodes where upon our heroes’ whiteboards unfolded about making an exciting new particle, “the axion” on Earth. Could axions be made inside an artificial Sun made by the National Ignition Facility? This summer, I was excited about this, but as you already know by now from reading tonight’s whiteboards, I made a terrible, terrible mistake.
To estimate the rate of axions I used the relative power produced by the Sun versus the small compressed sample produced at the National Ignition Facility. In both cases a material is made so hot that atoms are ripped apart into their constituents: electrons and nuclei. Such a gas is called a plasma, and plasma is sometimes called “the fourth state of matter” as it is step hotter than just ordinary gas. It is not unfamiliar. The glowing orange material in a neon sign is a plasma.

The Northern Lights are an example of a plasma you can see. (National Geographic) The last three episode's whiteboards explored if a dense hot plasma could make the elusive Axion particle.
I was comparing the large far-away plasma in the core of the Sun to the tiny, but close, plasma created in the lab. It initially looked like the laboratory won. To understand what was wrong, you first have to understand how the Sun produces its energy.
The strong nuclear interaction likes to bind protons and neutrons together. And Martha Stewart says, “It’s a good thing”. Without it, the only atoms we would ever have are hydrogen. If hydrogen were our only element, we’d have an unperiodic periodic table–with only one entry, hydrogen. In real life, the nucleus of every atom is held together by this force. And its strength is impressive. For example, in helium and every element heavier the protons are repelling each other. Same-sign charges (in the protons’ case, both positive) repel with a force increasing as the square of their distance from each other decreases. A nucleus is extremely small, and those protons are so close they want to fly apart, badly. The strong interaction overcomes this repulsion and nuclei stay bound. That’s why it is called the “strong interaction” (or “strong force”).
But there’s a wrinkle. If you try to bring just two together (either protons or neutrons) there is only one combination that is stable: a pairing of one neutron and one proton. The pairing of two protons or two neutrons is not.
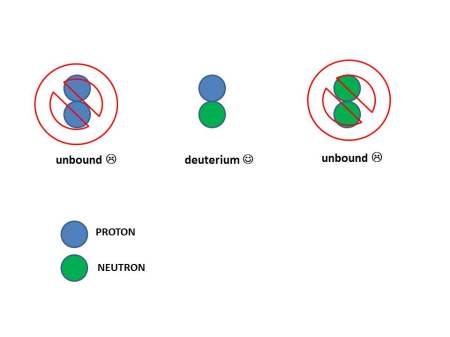
You might think you the strong force could combine any pairing of protons and neutrons. But quantum mechanics only allows a proton and neutron to bind. The result is heavy hydrogen, or “deuterium”.
It might seem the reason why two protons are not bound is because of their electric repulsion. But that would not explain why two neutrons are also not bound. The answer really lies within the constraints of the quantum mechanics of identical particles. It turns out that the only way to put two identical neutrons or protons together is if they have angular momentum, but then they are not bound. We teach our physics majors all about this at UCLA in our introductory quantum mechanics class. If you can take a quantum mechanics class, I highly recommend it.
The core of the Sun is full of protons but no free neutrons. So the only way to make energy from them is to convert one of those protons into a neutron so you can bind them. This bound state of a neutron and proton is still chemically hydrogen, but it has an extra neutron so it is called “heavy hydrogen”, or more technically deuterium. That’s the same “heavy” of “heavy water”. “Heavy” water is made with “heavy hydrogen”. But the reaction does not conserve electric charge so you need a light positively charged particle to fly away, and it turns out to be the anti-matter partner of an electron, which has a positive charge (e+), and so is a “positron”. But that introduces a new problem. A positron is a type of particle called “lepton” and for reasons not yet understood, you can’t vioate the number of leptons. So you also need a neutrino (ν) to be made as well to not create any net leptons. (These neutrinos were detected from the Sun over the last few decades. They changed our entire understanding of neutrinos but that’s a story for another day.) It’s easiest to see graphically:
Ultimately these deuteriums (deuteria?) undergo further reactions and the net reaction in the Sun is:
4 protons –> 1 helium nucleus (2 protons + 2 neutrons) + 2 neutrinos + lots of energy.
The rate of energy production in the Sun is so slow that pound-for-pound you produce more energy than the Sun. Just sitting in front of your computer, digesting your last meal, you produce about 1 Watt of power per kilogram of your body weight. The sun produces only about 0.0002 Watts per kilogram. The Sun is just bigger. A lot bigger. While it is tempting to think of it as a massive nuclear furnace, it really is just smoldering. We’re lucky too. If the Sun’s reactions were not throttled by the weak interaction we would be living next to a nuclear bomb, not a star.
But the good people at the National Ignition Facility cannot wait around for such slow reactions. Instead, they use heavy hydrogen (deuterium). and an even heavier hydrogen with two neutrons and proton, or “tritium”. Their net reaction is:
deuterium+ tritium -> helium + neutron + energy.
No neutrons or protons changed their identity. They just change who they hang out with. This proceeds by the strong interactiona nd also releases massive energy. This reaction is about 1025 times faster than the proton+proton fusion in the Sun. And there’s the rub. You can’t compare the two Sun’s directly. The boys’ calculation was off by “only” a factor of 1025.
Before the taping of tonight’s episode, many of the crew members asked me why there was an unhappy face ![]() at the end of one of the whiteboards. Now you know why.
at the end of one of the whiteboards. Now you know why.
2012-11-15 09:05:19
Source: https://thebigblogtheory.wordpress.com/2011/09/30/s05e03-the-pulled-groin-extrapolation/
Source:



