| Visitors Now: | |
| Total Visits: | |
| Total Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Negative Absolute Temperature for Motional Degrees of Freedom
From
Absolute temperature is usually bound to be positive. Under special conditions, however, negative temperatures—in which high-energy states are more occupied than low-energy states—are also possible. Such states have been demonstrated in localized systems with finite, discrete spectra. Here, we prepared a negative temperature state for motional degrees of freedom. By tailoring the Bose-Hubbard Hamiltonian, we created an attractively interacting ensemble of ultracold bosons at negative temperature that is stable against collapse for arbitrary atom numbers. The quasimomentum distribution develops sharp peaks at the upper band edge, revealing thermal equilibrium and bosonic coherence over several lattice sites. Negative temperatures imply negative pressures and open up new parameter regimes for cold atoms, enabling fundamentally new many-body states.
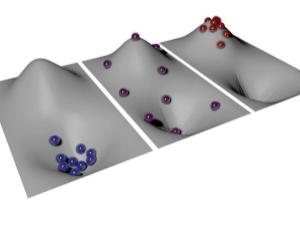
Temperature depends on the energy landscape (Image: Ludwig Maximilian University of Munich)
According to temperature's entropic definition, the highest positive temperature possible corresponds to the most disordered state of the system. This would be an equal number of particles at every point on the landscape. Increase the energy any further and you'd start to lower the entropy again, because the particles wouldn't be evenly spread. As a result, this point represents the end of the positive temperature scale.
In principle, though, it should be possible to keep heating the particles up, while driving their entropy down. Because this breaks the energy-entropy correlation, it marks the start of the negative temperature scale, where the distribution of energies is reversed – instead of most particles having a low energy and a few having a high, most have a high energy and just a few have a low energy. The end of this negative scale is reached when all particles are at the top of the energy hill.
See more and subscribe to NextBigFuture at 2013-01-04 10:00:52 Source: http://nextbigfuture.com/2013/01/negative-absolute-temperature-for.html
Source: