| Visitors Now: | |
| Total Visits: | |
| Total Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Lithium-sulfur battery breakthroughs for holding good charges for up to 200 recharges
Two recently published papers, both originating from the lab of Hector Abruña, the Emile M. Chamot Professor of Chemistry and Chemical Biology, describe breakthroughs in the durability and performance of lithium-sulfur battery cathodes, one by using a component of corn starch, and the other, by modeling a nanocomposite material after the yolk-shell structure of eggs.
“Lithium-sulfur batteries could potentially offer about five times the energy density of today’s typically used lithium-ion batteries,” said Yingchao Yu, Ph.D. student with Abruña, and co-first author on the JACS publication. “But a lithium-sulfur battery is not a stable system, as its capacity tends to fade over a short period of time.”
After about 50 charge cycles, the energy density of a lithium-sulfur battery decreases rapidly due to a phenomenon called the polysulfide shuttling effect, which is when the polysulfide chains in the battery’s cathode (positive end) dissolve in the electrolyte, the ionizing liquid that allows electrons to flow.
To combat this problem and stabilize the sulfur, the researchers used amylopectin, a polysaccharide that’s a main component of corn starch.
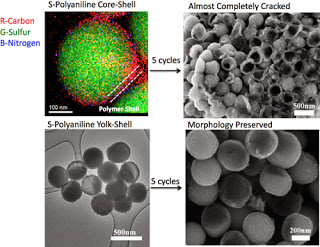
Top left, false-colored energy dispersive X-ray mapping of a sulfur-polyaniline core-shell nanocomposite, next to a scanning electron microscopy image of the core shells cracked after five cycles. Bottom left is a transmission electron microscopy image of a yolk-shell structure coating with polyaniline, and, right, its preserved morphology after five charge cycles.
Source: http://nextbigfuture.com/2013/10/lithium-sulfur-battery-breakthroughs.html