

| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Artificial Photosynthesis Advances: Transition Metals Turned into Practical Catalyst for Solar, Other Applications
The lab of Kenton Whitmire, a Rice professor of chemistry, teamed up with researchers at the University of Houston and discovered that growing a layer of an active catalyst directly on the surface of a light-absorbing nanorod array produced an artificial photosynthesis material that could split water at the full theoretical potential of the light-absorbing semiconductor with sunlight.
Scientists at Rice University and the University of Houston created a catalyst from three elements — iron, manganese and phosphorus — and then coated it evenly onto an array of titanium dioxide nanorods to create a highly efficient photoanode for artificial photosynthesis.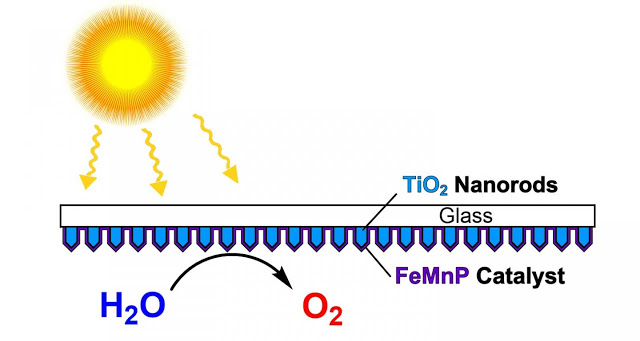
Credit: Whitmire Research Group/Rice University
The Rice team came up with a way to combine three of the most abundant metals — iron, manganese and phosphorus — into a precursor that can be deposited directly onto any substrate without damaging it.
To demonstrate the material, the lab placed the precursor into its custom chemical vapor deposition (CVD) furnace and used it to coat an array of light-absorbing, semiconducting titanium dioxide nanorods. The combined material, called a photoanode, showed excellent stability while reaching a current density of 10 milliamps per square centimeter, the researchers reported.
The results appear in two new studies. The first, on the creation of the films, appears in Chemistry: A European Journal. The second, which details the creation of photoanodes, appears in ACS Nano.
Whitmire said the catalyst is grown from a molecular precursor designed to produce it upon decomposition, and the process is scalable. The Rice lab combined iron, manganese and phosphorus (FeMnP) into a molecule that converts to a gas when vacuum is applied. When this gas encounters a hot surface via CVD, it decomposes to coat a surface with the FeMnP catalyst.
A photo shows an array of titanium dioxide nanorods with an even coating of an iron, manganese and phosphorus catalyst. The combination developed by scientists at Rice University and the University of Houston is a highly efficient photoanode for artificial photosynthesis.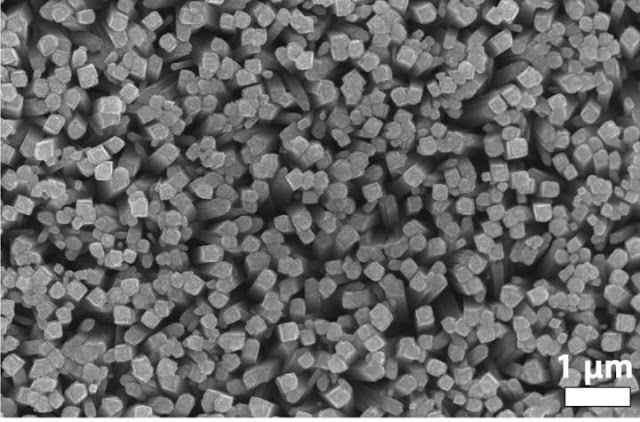
Credit: Whitmire Research Group/Rice University
The researchers claim their film is “the first heterobimetallic phosphide thin film” created from iron, manganese and phosphorus that starts out as a single precursor. The resulting films contain stable hexagonal arrays of atoms that, until now, had only been seen at temperatures above 1,200 degrees Celsius. The Rice films were created at 350 degrees C in 30 minutes.
The researchers coated the three-dimensional arrays of titanium dioxide nanorods with the metallic-looking film. The composite material showed potential as a high-surface-area semiconductor for photoelectrochemical cells.
Growing the transition metal coating directly onto the nanorods allows for maximum contact between the two, Whitmire said. “That metallic, conductive interface between the semiconductor and the active catalytic surface is key to the way this device works,” he said.
The film also has ferromagnetic properties, in which the atoms’ magnetic moments align in the same direction. The film has a low Curie temperature, the temperature at which some materials’ magnetic properties need to be induced. That could be useful for magnetic refrigeration, the researchers said.
Having established their technique, Whitmire said it will now be much easier to investigate hybrid catalysts for many applications, including petrochemical production, energy conversion and refrigeration.
“It seems like when it rains, it pours,” he said. “We spent a very long time putting everything together, and now all of a sudden there are too many things to do.”
Read the Chemistry abstract at http://onlinelibrary.wiley.com/doi/10.1002/chem.201700203/full.
Read the ACS Nano abstract at http://pubs.acs.org/doi/abs/10.1021/acsnano.7b00704
Source: http://www.ineffableisland.com/2017/03/artificial-photosynthesis-advances.html


