

| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Graphene used in a molecule that converts carbon dioxide to carbon monoxide
An international team of scientists has designed a molecule that uses light or electricity to convert the greenhouse gas carbon dioxide into carbon monoxide. The process includes using a nanographene-rhenium complex connected via an organic compound known as bipyridine to trigger a highly efficient reaction that could someday replace solar cells.
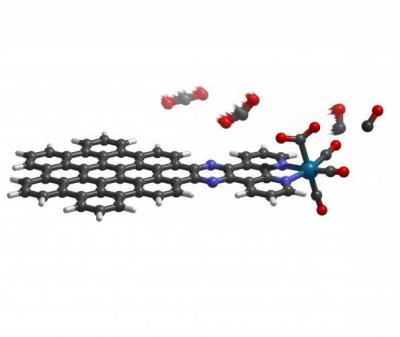
The molecule acts as a two-part system: a nanographene “energy collector” that absorbs energy from sunlight and an atomic rhenium “engine” that produces carbon monoxide. The energy collector drives a flow of electrons to the rhenium atom, which repeatedly binds and converts the normally stable carbon dioxide to carbon monoxide. The idea to link nanographene to the metal arose from earlier efforts to create a more efficient solar cell with the carbon-based material. But this model actually eliminates the solar cells, and uses the light-absorbing quality of nanographene alone to drive the reaction.
Source: http://www.graphene-info.com/graphene-used-molecule-converts-carbon-dioxide-carbon-monoxide


