| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Self-Healing Smartphones on the Horizon
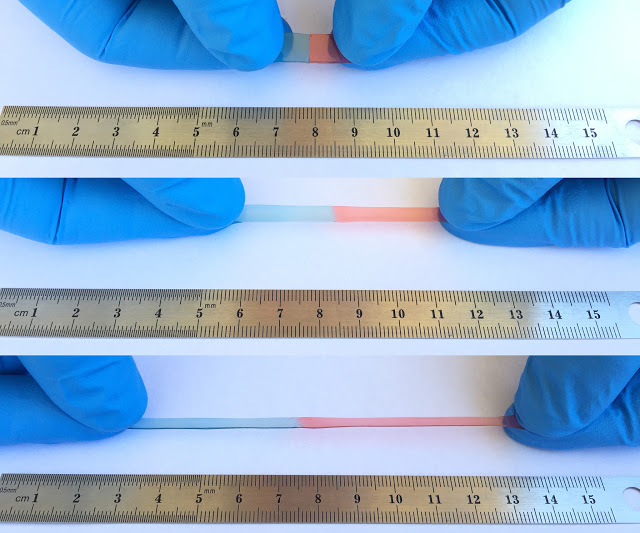
“When I was young, my idol was Wolverine from the X-Men,” Chao Wang, Ph.D., says. “He could save the world, but only because he could heal himself. A self-healing material, when carved into two parts, can go back together like nothing has happened, just like our human skin. I’ve been researching making a self-healing lithium ion battery, so when you drop your cell phone, it could fix itself and last much longer.”A new material not only heals itself, but it also stretches up to 50 times its usual size; these properties could fix your phone’s battery if it cracks or prevent it from breaking in the first place.
Wang’s team at the University of California, Riverside, turned instead to a different type of non-covalent bond called an ion-dipole interaction, a force between charged ions and polar molecules. “Ion-dipole interactions have never been used for designing a self-healing polymer, but it turns out that they’re particularly suitable for ionic conductors,” Wang says. The key design idea in the development of the material was to use a polar, stretchable polymer, poly(vinylidene fluoride-co-hexafluoropropylene), plus a mobile, ionic salt. The polymer chains are linked to each other by ion-dipole interactions between the polar groups in the polymer and the ionic salt.
The resulting material could stretch up to 50 times its usual size. After being torn in two, the material automatically stitched itself back together completely within one day.
As a test, the researchers generated an “artificial muscle” by placing a non-conductive membrane between two layers of the ionic conductor. The new material responded to electrical signals, bringing motion to these artificial muscles, so named because biological muscles similarly move in response to electrical signals (though Wang’s materials are not intended for medical applications).
For the next step, the researchers are working on altering the polymer to improve the material’s properties. For example, they are testing the material in harsh conditions, such as high humidity. “Previous self-healing polymers haven’t worked well in high humidity, Wang says. “Water gets in there and messes things up. It can change the mechanical properties. We are currently tweaking the covalent bonds within the polymer itself to get these materials ready for real-world applications.”
Wang acknowledges funding from start-up funds from the University of California, Riverside.
The researchers presented their work today at the 253rd National Meeting & Exposition of the American Chemical Society (ACS). ACS, the world’s largest scientific society, is holding the meeting in San Francisco through Thursday. It features more than 14,000 presentations on a wide range of science topics.
Katie Cottingham, Ph.D.
The American Chemical Society
Source: http://www.ineffableisland.com/2017/04/self-healing-smartphones-on-horizon.html