| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
A Look into the Deep Interiors of Stars and Planets
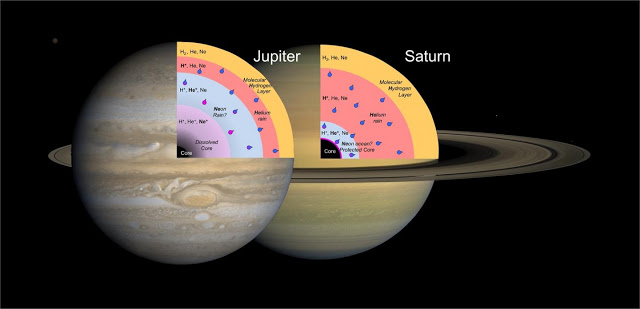
Alexander Goncharov, Stewart McWilliams, and the rest of the team’s work on noble gases could help solve the mystery of why Saturn emits more heat from its interior than would be expected. In Jupiter and Saturn, helium would be insulating near the surface and turn metal-like at depths close to both planet’s cores, where it is also predicted to be dissolved in hydrogen. But neon behaved differently in the laboratory conditions mimicking the two gas giants. In Saturn, it would remain an insulator even at the core. As such, an ocean-like envelope of undissolved neon could collect deep within the planet and prevent the erosion of Saturn’s core compared to its neighbor, Jupiter.
Credit: University of Edinburgh (graphics), NASA (photos)
New work from a team of scientists led by Carnegie’s Alexander Goncharov used laboratory techniques to mimic stellar and planetary conditions, and observe how noble gases behave under these conditions, in order to better understand the atmospheric and internal chemistry of these celestial objects. Their work is published the week of June 15 by Proceedings of the National Academy of Sciences.
The gases are called “noble” due to a kind of chemical aloofness; they normally do not combine, or “react,” with other elements. Of particular interest were changes in the gases’ ability to conduct electricity as the pressure and temperature changed, because this can provide important information about the ways that the noble gases do actually interact with other materials in the extreme conditions of planetary interiors and stellar atmospheres.
Insulators are materials that are unable to conduct the flow of electrons that make up an electric current. Conductors, or metals, are materials that can maintain an electrical current. Nobel gases are not normally conductive at ambient pressures, but conductivity can be induced under higher pressures.
The research team–which included Carnegie’s Stewart McWilliams (the lead author), and Douglas Allen Dalton, as well as Mohammad Mahmood of Howard University and Zuzana Konopkova of Deutsches Elektronen-Synchrotron Photon Science in Hamburg, Germany–found that helium, neon, argon, and xenon transform from visually transparent insulators to visually opaque conductors under varying extreme conditions that mimic the interiors of different stars and planets.
This has several exciting implications for how noble gases behave in the atmospheres and interiors of planets and stars.
For example, it could help solve the mystery of why Saturn emits more heat from its interior than would be expected given its stage of formation. This is tied to the ability, or inability, of the noble gases to be dissolved in the liquid hydrogen present in abundance in the interior of gas giant planets such as Saturn and Jupiter.
In Jupiter and Saturn, helium would be insulating near the surface and turn metal-like at depths close to both planet’s cores. The change from insulator to metal occurs under pressure and temperature conditions at which hydrogen–the main constituent of these planets–is also known to be metallic. It is predicted that helium is, in fact, dissolved in hydrogen under these conditions on both planets and, furthermore, that the miscibility–or ability of two substances to mix–of hydrogen-helium mixtures is correlated with this kind of insulator-to-metal transformation.
However, there was an observed difference in the behavior of neon between the laboratory conditions mimicking the two gas giants. The team’s results indicate that neon would remain an insulator even in Saturn’s core. As such, an ocean-like envelope of undissolved neon could collect deep within the planet and prevent the erosion of Saturn’s core compared to its neighbor Jupiter, where core materials, such as iron, would be dissolving into the surrounding liquid hydrogen.
This lack of core erosion could potentially explain why Saturn is giving off so much internal heat compared to its neighbor Jupiter. Erosion of a planet’s core, as in Jupiter, leads to planetary cooling as dense matter is raised upward during mixing, converting heat to gravitational potential energy, whereas in Saturn denser material is allowed to collect at the center of the planet, producing hotter conditions. The fact that Saturn gives off a great deal of internal heat has been a longstanding mystery. These findings could provide the key to solving it.
Another implication of the team’s findings involves white dwarf stars, which are the collapsed remnants of once-larger stars, having about the mass of our Sun. They are very compact, but have faint luminosities as they give off residual heat. Dense helium is known to exist in the atmospheres of white dwarf stars and may form the surface atmosphere of some of these celestial bodies. The conditions simulated by the team’s laser-heated diamond-anvil cell indicate that this stellar helium should be more opaque (and conducting) than previously expected and this opacity could slow the cooling rates of helium-rich white dwarfs, as well as affect their color.
“Our findings provide yet another example of the vast array of applications for extreme pressure research,” Goncharov said. “Further research could reveal so much more about what’s going on in the interiors of these objects that are too distant for us to observe more directly.”
Contacts and sources:
Alex Goncharov
Source:





how do you know that have you been there
have you been there 







It’s funny how people accept pictures.
2 buttons Real or Fake News
you don’t need to leave a message
Researchers have only begun to scratch the surface of the complexities contained in God’s creation.