

| Visitors Now: | |
| Total Visits: | |
| Total Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
What Came First the Chicken or the Egg? Super Computer Gives Partial Answer-Chicken Protein
Sunday, July 11, 2010 6:27
% of readers think this story is Fact. Add your two cents.
Researchers at the University of Warwick and the University of Sheffield have applied computing power to crack a problem in egg shell formation. The work may also give a partial answer to the age old question “what came first the chicken or the egg?”
The answer to the question in this context is “chicken” or – at least a particular chicken protein. There is however a further twist in that this particular chicken protein turns out to come both first and last. That neat trick it performs provides new insights into control of crystal growth which is key to egg shell production.

Image credit: University of Warwick
Researchers had long known that a chicken eggshell protein called ovocledidin-17 (OC-17) must play some role in egg shell formation. The protein is found only in the mineral region of the egg (the hard part of the shell) and lab bench results showed that it appeared to influence the transformation of (CaCo3) into calcite crystals. The mechanism of this control remained unclear. How this process could be used to form an actual eggshell remained unclear.
University of Warwick researchers Mark Rodger and David Quigley, in collaboration with colleagues at the University of Sheffield, have now been able to apply a powerful computing tool called metadynamics and the UK national supercomputer in Edinburgh to crack this egg problem.

Image credit: University of Warwick
Dr David Quigley from the Department of Physics and Centre for Scientific Computing, University of Warwick, said: “Metadynamics extends conventional molecular dynamics (MD) simulations and is particularly good at sampling transitions between disordered and ordered states of matter.”
Using these tools The Warwick and Sheffield researchers were able to create simulations that showed exactly how the protein bound to amorphous calcium carbonate surface using two clusters of “arginine residues”, located on two loops of the protein and creating a literal chemical “clamp” to nano sized particles of calcium carbonate.
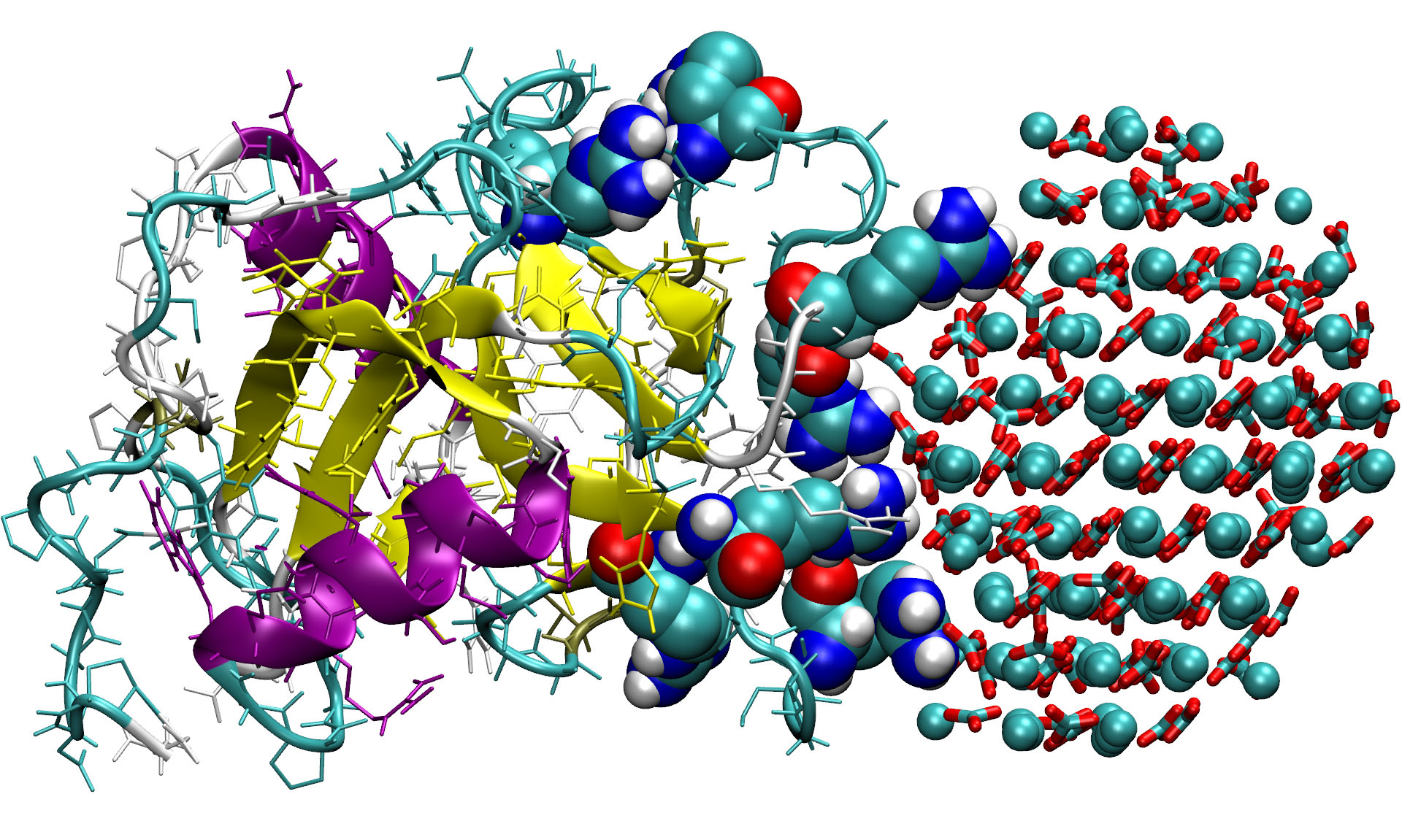
Image credit: University of Warwick
While clamped in this way, the OC-17 encourages the nanoparticles of calcium carbonate to transform into “calcite crystallites” that form the tiny of nucleus of crystals that can continue to grow on their own. But they also noticed that sometimes this chemical clamp didn’t work. The OC-17 just seemed to detatch from the nanoparticle or “be desorbed”.
Professor Mark Rodger from Department of Chemistry and Centre for Scientific Computing, University of Warwick, said “With the larger nanoparticles we examined we found that the binding sites for this chemical clamp were the same as the smaller nanoparticles but the binding was much weaker. In the simulations we performed, the protein never desorbed from the smaller nanoparticle, but always fell off or desorbed from the larger one. However in each case, desorption occurred at or after nucleation of calcite.”
The researchers had therefore uncovered an incredibly elegant process allowing highly efficient recycling of the OC-17 protein. Effectively it acts as a catalyst, clamping on to calcium carbonate particles to kickstart crystal formation and then dropping off when the crystal nucleus is sufficiently large to grow under its own steam. This frees up the OC-17 to promote more yet more crystallization, facilitating the speedy, literally overnight creation of an egg shell.
The researchers believe that this new insight into the elegant and highly efficient methods of promoting and controlling crystallization in nature will be of great benefit to anyone exploring how to promote and control artificial forms of crystallization.
Contacts and sources:
University of Warwick News Release
Peter Dunn, Head of Communications
Communications Office, University House,
University of Warwick, Coventry, CV4 8UW, United Kingdom
Citation: The paper entitled “Structural Control of Crystal Nuclei by an Eggshell Protein” by Colin L. Freeman , John H. Harding , David Quigley , and Professor P. Mark Rodger, is published in Angewandte Chemie International Edition, DOI: 10.1002/anie.201000679 www3.interscience.wiley.com/journal/123506601/abstract
Professor Mark Rodger Department of Chemistry
and Centre for Scientific Computing , University of Warwick
Tel: +44 (0)24 76 523239
Dr David Quigley, Department of Physics and
Centre for Scientific Computing, University of Warwick
44 (0)2476 574580


