

| Online: | |
| Visits: | |
| Stories: |

| Story Views | |
| Now: | |
| Last Hour: | |
| Last 24 Hours: | |
| Total: | |
Rapidly Domesticate Wild Edible Plants with Gene Editing, Feed the World with New Farmable Crops Say Researchers
“In theory, you can now take those traits that have been selected for over thousands of years of crop domestication–such as reduced bitterness and those that facilitate easy harvest–and induce those mutations in plants that have never been cultivated,” says senior author Michael Palmgren, a botanist who heads an interdisciplinary think tank called “Plants for a Changing World” at the University of Copenhagen.
This figure shows how during the domestication of ancestral crops, plants carrying spontaneous mutations in domestication genes were selected for. The same genes can be targeted in wild plants by genome editing, resulting in a rapidly domesticated plant.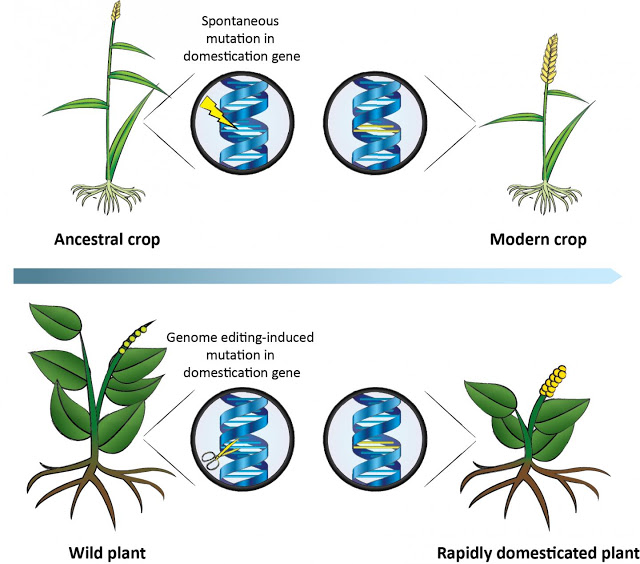
Credit: Palmgren et al./Trends in Plant Science 2017
The approach has already been successful in accelerating domestication of undervalued crops using less precise gene-editing methods. For example, researchers used chemical mutagenesis to induce random mutations in weeping rice grass, an Australian wild relative of domestic rice, to make it more likely to hold onto its seeds after ripening. And in wild field cress, a type of weedy grass, scientists silenced genes with RNA interference involved with fatty acid synthesis, resulting in improved seed oil quality.
This strategy also has potential to address problems related to pesticide use and the impact of large-scale agriculture on the environment. For example, runoff from excess nitrogen in fertilizers is a common pollutant; however, wild legumes, through symbiosis with bacteria, can turn nitrogen available in the atmosphere into their own fertilizer. “Why not try to domesticate more of these plants?” Palmgren says.
Accelerating domestication could face similar ethical, economic, and legal issues that arise whenever the gene editing of crops is involved. However, public opinion may differ somewhat because this approach doesn’t involve taking a gene from another organism but rather deleting existing genes. For farmers and breeders, adding underutilized species of plants may not be immediately attractive because there is less of a demand, and so work on building consumer’s appetite for them will be necessary. However, the public good of making such a switch could in the end be a selling point.
Palmgren’s group, which aims to evaluate new directions for agriculture, published a related paper two years ago on using gene editing to make domesticated plants more “wild” and thus hardier for organic farmers (doi: 10.1016/j.tplants.2015.04.011). They hope that as agriculture evolves to meet increasing demand, they can help prepare the public and policymakers for the implementation of genome editing into our food supply.
This work was supported by the University of Copenhagen Excellence Programme for Interdisciplinary Research.
Source: http://www.ineffableisland.com/2017/03/rapidly-domesticate-wild-edible-plants.html


